Polar Vs Nonpolar Molecules Polar and nonpolar molecules are the two broad classes of molecules Polarity describes the distribution of electrical charge around a molecule Charge is evenly distributed
To make you understand how polar and nonpolar are different from each other here are some major differences between polar and nonpolar The molecules with polar bonds are usually asymmetrical The molecules with non polar bonds are usually symmetrical It has electrical poles It does not have electrical poles Nonpolar molecules are the molecules in which the electrons are equally shared among the involved atoms and have a zero dipole moment A net dipole moment is present in polar molecules
Polar Vs Nonpolar Molecules

Polar Vs Nonpolar Molecules
https://i.ytimg.com/vi/GfW4hVEiW9U/maxresdefault.jpg
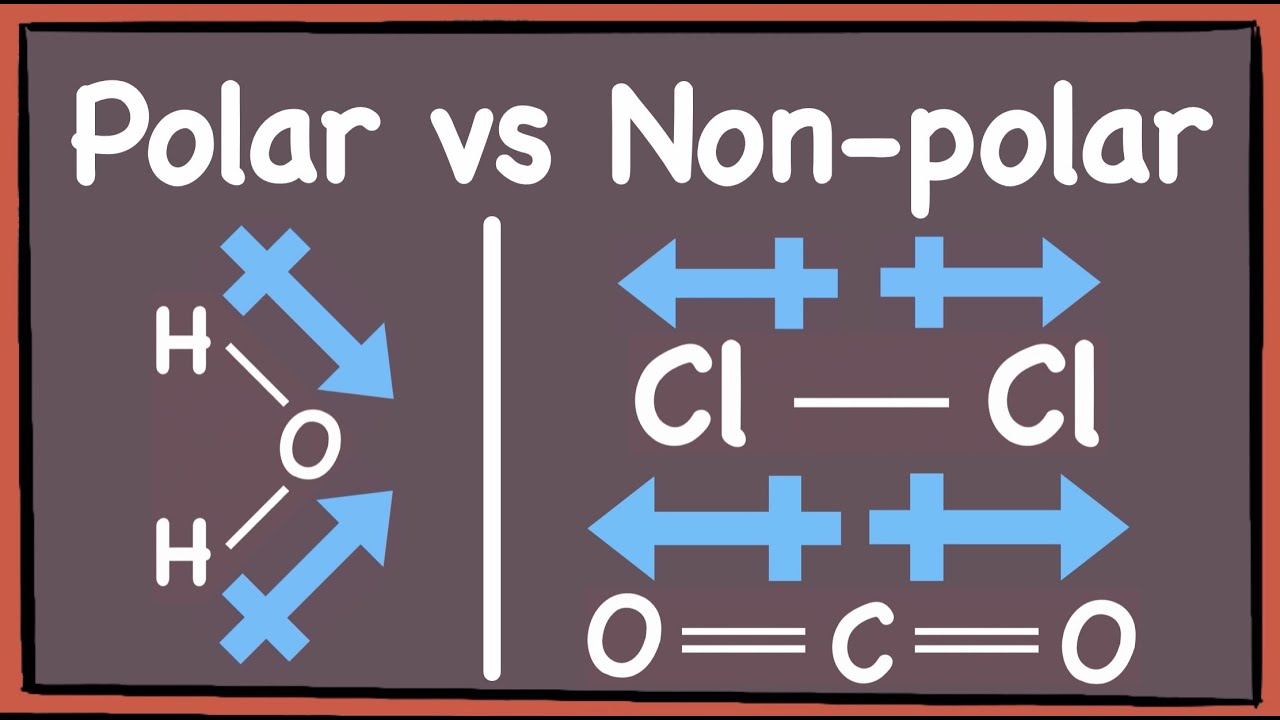
Polar Vs Nonpolar Molecules How To Tell GCE A Level Chemistry YouTube
https://i.ytimg.com/vi/WkiA4Du-5oE/maxresdefault.jpg

Non polar And Polar Covalent Bonds Ppt Download
https://slideplayer.com/slide/15837319/88/images/11/Contrasting+Nonpolar+vs+Polar+Molecules.jpg
Polar Molecules A polar molecule is usually formed when the one end of the molecule is said to possess more positive charges and whereas the opposite end of the molecule has negative charges creating an electrical pole Non polar molecules are symmetric with no unshared electrons Polar molecules are asymmetric either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded
The main difference between polar and nonpolar molecules is net dipole moment The net dipole moment is formed on the atoms of polar molecules but not on non polar molecules A polar molecule is a chemical compound with an unequal electron distribution between the atoms resulting in a dipole moment Polarity refers to the difference between a molecule s electrical poles that indicates how polar the molecule is
More picture related to Polar Vs Nonpolar Molecules
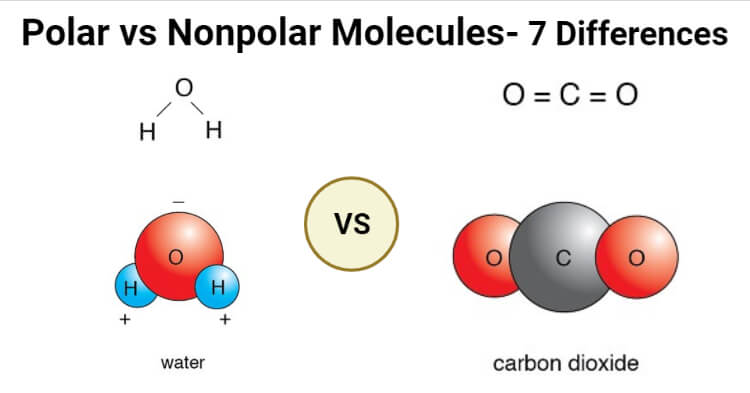
Anupama Sapkota
https://scienceinfo.com/wp-content/uploads/2021/06/Polar-vs-Nonpolar-Molecules.jpeg
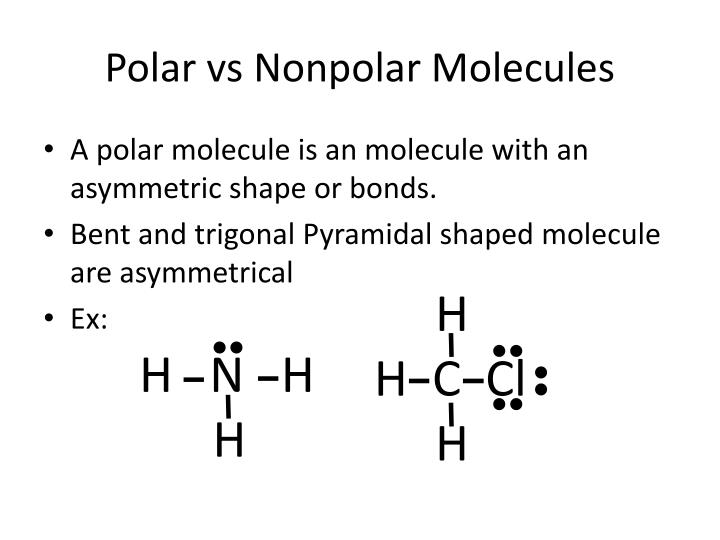
PPT Polar Or Nonpolar PowerPoint Presentation ID 3483667
https://image1.slideserve.com/3483667/polar-vs-nonpolar-molecules-n.jpg

PolarNonPolarMolecules Pathways To Chemistry
http://www.pathwaystochemistry.com/wp-content/uploads/PolarNonPolarMolecules-1.jpg
Nonpolar molecules have an even distribution of electrons while polar molecules have an uneven distribution In this article we will explore the attributes of nonpolar and polar molecules and compare their properties Nonpolar molecules form either when electrons are equally shared between atoms in a molecule or when the arrangement of electrons in a molecule is asymmetrical so that dipole charges cancel each other out
[desc-10] [desc-11]
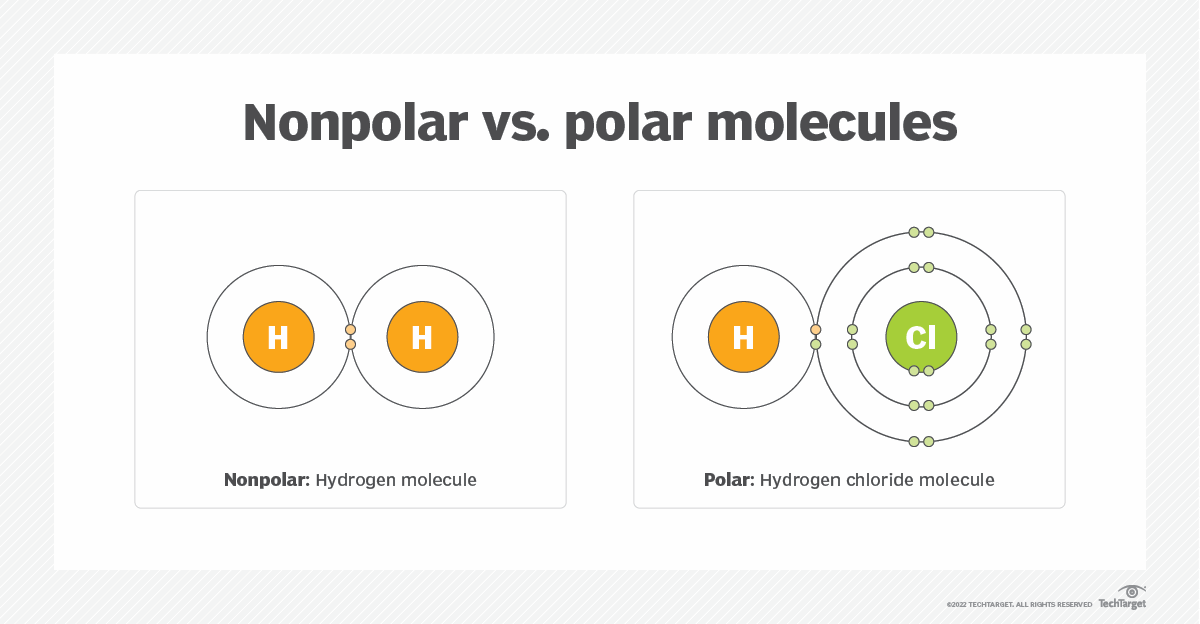
What Is Polarity Definition From TechTarget
https://cdn.ttgtmedia.com/rms/onlineimages/nonpolar_vs_polar_molecules-h.png

Pin On Science
https://i.pinimg.com/originals/40/21/0d/40210d4cae2e79084de8d5405f3520b1.png

https://sciencenotes.org › polar-and-nonpolar-molecules
Polar and nonpolar molecules are the two broad classes of molecules Polarity describes the distribution of electrical charge around a molecule Charge is evenly distributed

https://byjus.com › chemistry › difference-between-polar-and-non-polar
To make you understand how polar and nonpolar are different from each other here are some major differences between polar and nonpolar The molecules with polar bonds are usually asymmetrical The molecules with non polar bonds are usually symmetrical It has electrical poles It does not have electrical poles
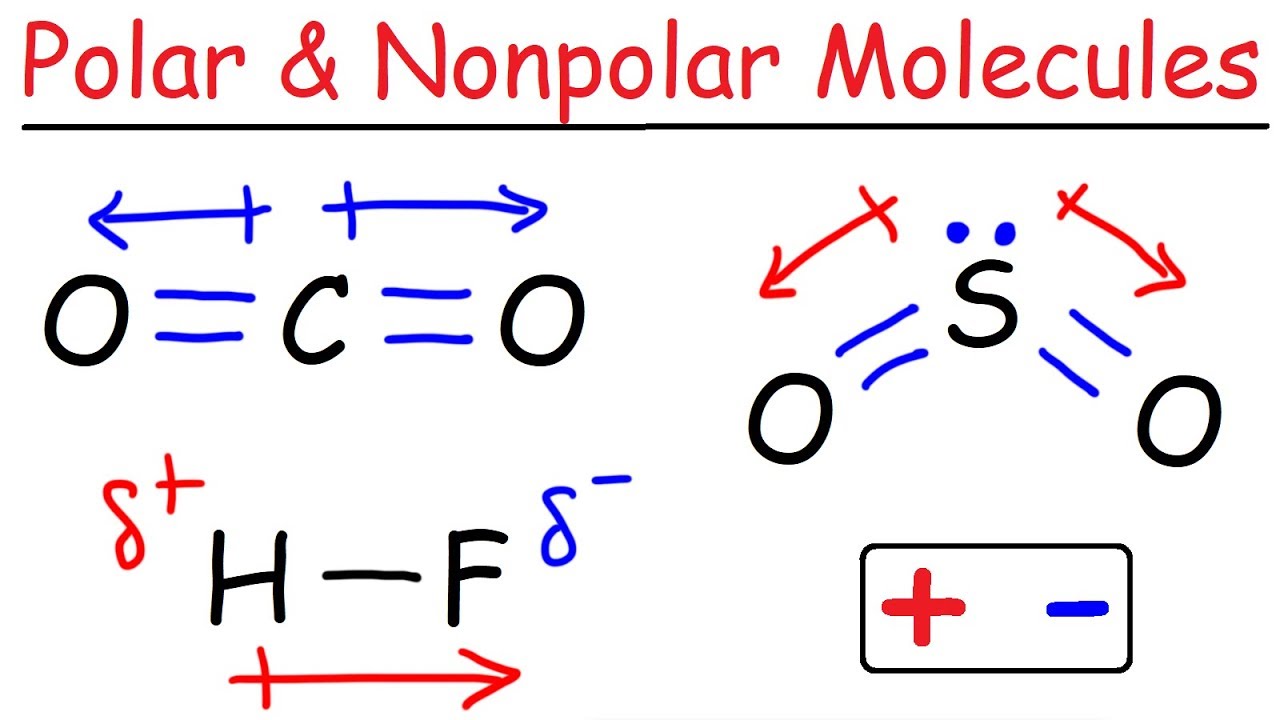
Polarity Of Molecules Quiz

What Is Polarity Definition From TechTarget

Methane Polar Or Nonpolar
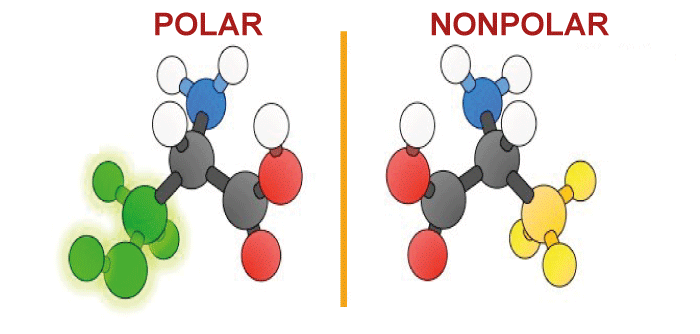
Nonpolar Molecule
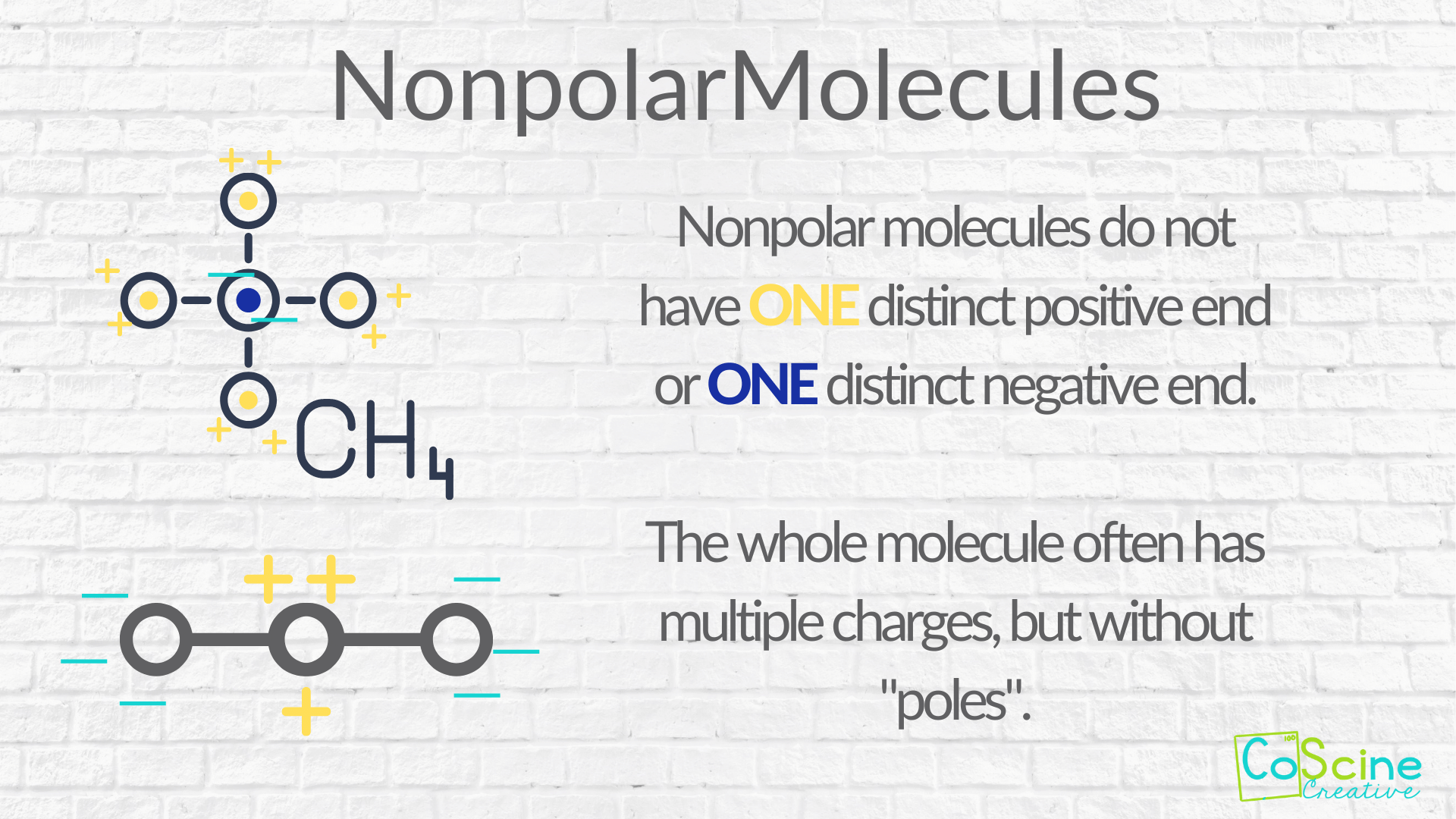
Nonpolar Molecule

Polar Vs Nonpolar Bonds Overview Examples Expii

Polar Vs Nonpolar Bonds Overview Examples Expii

Polar Vs Nonpolar Bonds Overview Examples Expii
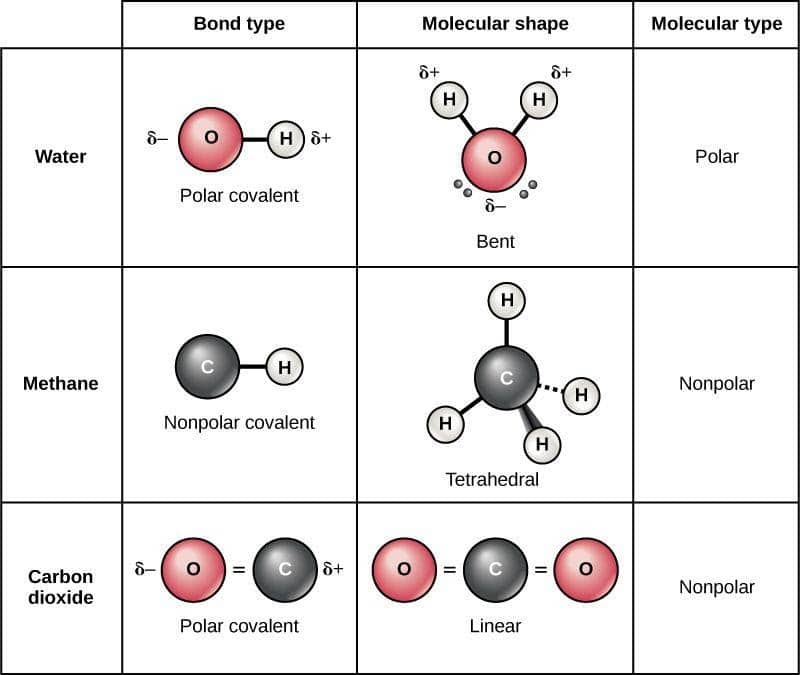
Polar Molecule Definition
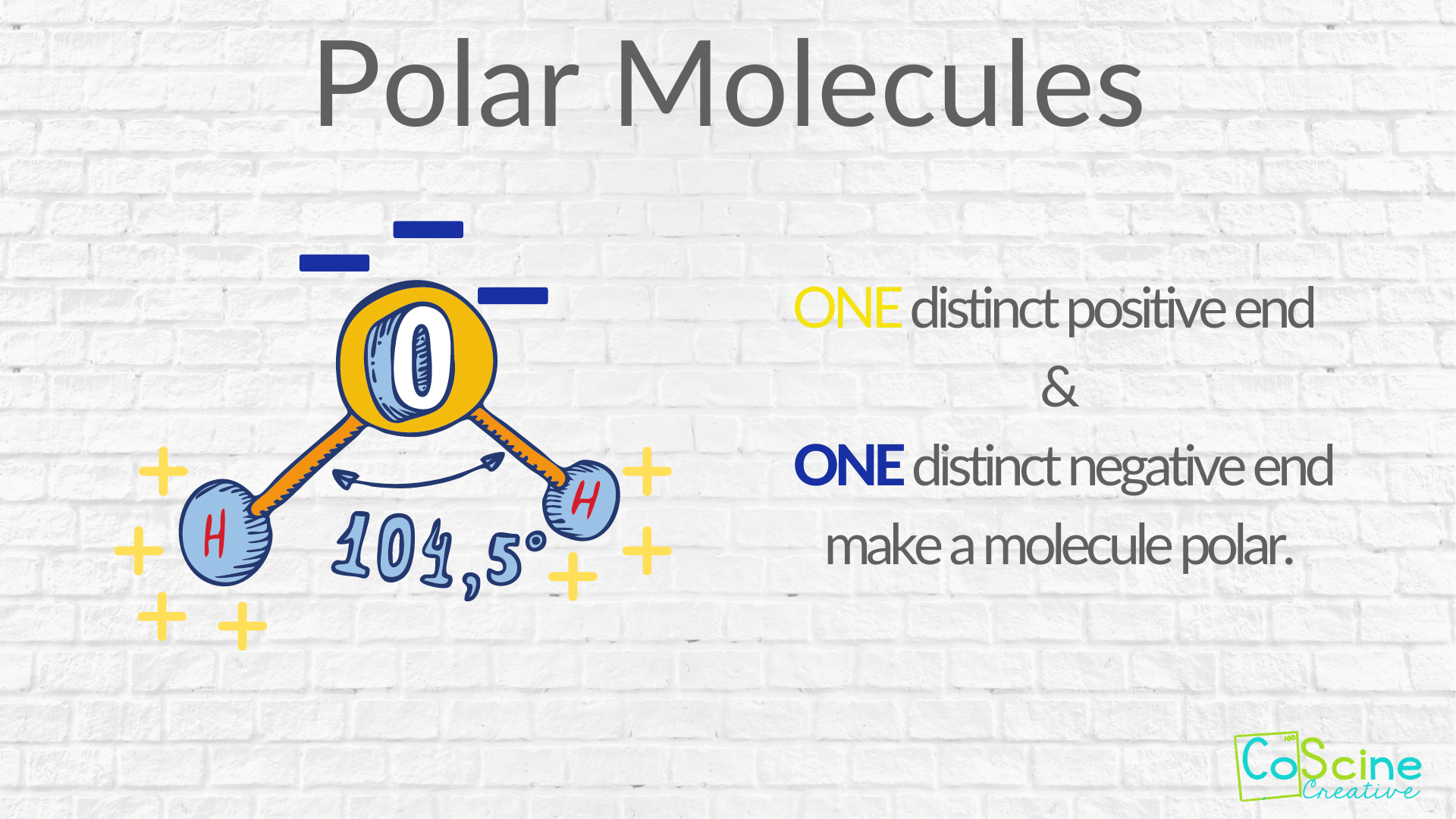
Polar Molecule Definition
Polar Vs Nonpolar Molecules - A polar molecule is a chemical compound with an unequal electron distribution between the atoms resulting in a dipole moment Polarity refers to the difference between a molecule s electrical poles that indicates how polar the molecule is