What Is Reduction In Chemistry Reduction means a gain of electrons which is what non metals do in order to observe the octet rule For example elemental chlorine has an oxidation number of 0
Write out a number line The original E red was 0 8277 V when in reference to the Standard Hydrogen Electrode which is normally the one at 0 0000 V If we Haber process is used to make ammonia The Haber process is the process in which ammonia is made by combining nitrogen and hydrogen with the use of an iron catalyst
What Is Reduction In Chemistry

What Is Reduction In Chemistry
https://i.ytimg.com/vi/0Mka43YwOPA/maxresdefault.jpg
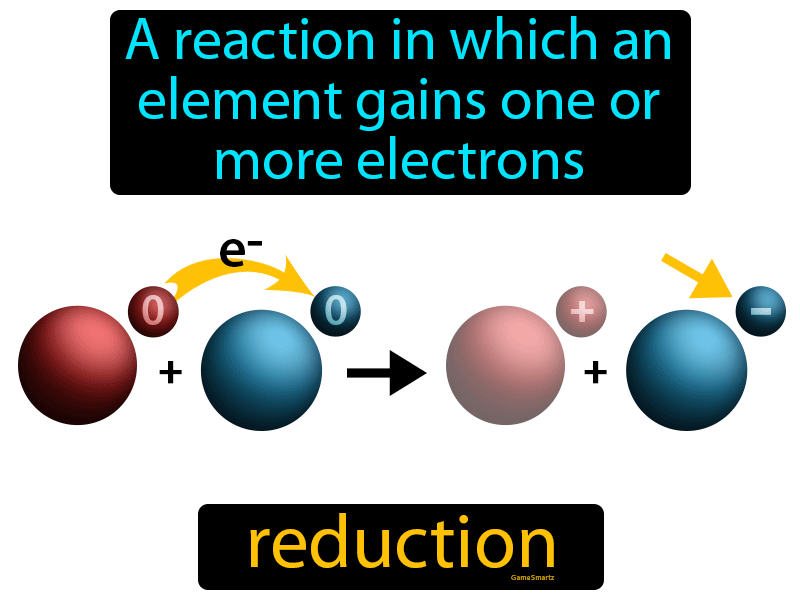
Reduction Easy To Understand
https://gamesmartz.com/upload/subjects/science/800/reduction.png

OXIDATION REDUCTION BIOCHEMISTRY
http://syafinasaslan.weebly.com/uploads/6/1/1/7/61176361/9203513_orig.png
Reduction is the loss of oxygen Oxidation is loss of electrons Reduction is gain of electrons Since oxygen is a highly electronegative element most of the electron density See below Looking at the notation you posted in the link I can say that Ba s is oxidized to Ba 2 aq Pb 2 aq is reduced to Pb s Anode is where oxidation occurs
I am assuming we have vanadium V as VO 2 and vanadium IV as VO 2 List the 1 2 equations in order least positive to most positive The half equation method features the loss or or gain of electrons as ACTUAL particles in order to balance redox equations Let s represent the oxidation of aluminum to give
More picture related to What Is Reduction In Chemistry
Illustrated Glossary Of Organic Chemistry Reduction Reaction
https://www.chem.ucla.edu/~harding/IGOC/R/reduction_reaction01.png

Standard Potential
https://mccord.cm.utexas.edu/chembook/pics/std-pots-shortlist.png

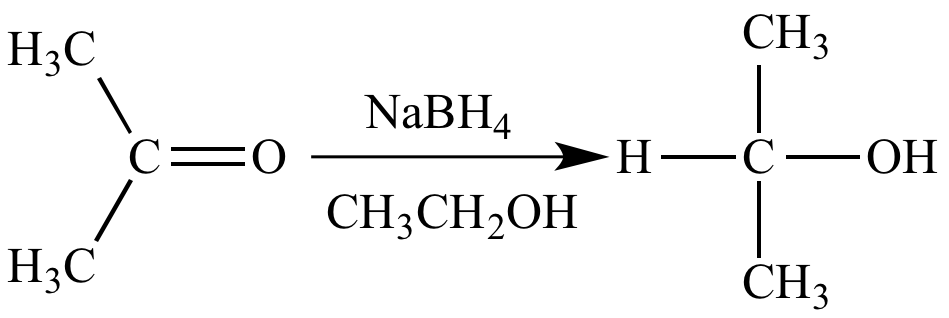
Esters To Alcohols Chemistry Steps
https://www.chemistrysteps.com/wp-content/uploads/2020/01/LiAlH4-Ester-reduction-Mechanism-.png
In a steel lead lined vault and the area around the vault should be guarded by armed guards and dogs Usually powerful oxidants should be stored in a dedicated cabinet There s no real difference between the oxidation number method and the half reaction method They are just different ways of keeping track of the electrons transferred
[desc-10] [desc-11]

State The Difference Between Oxidation And Reduction Brainly in
https://hi-static.z-dn.net/files/dc6/5a2c1165dbcb165d631dfe6bada98d28.jpg

Difference Between Oxidation And Reduction Oxidizing Agent And
https://i.pinimg.com/originals/71/ac/20/71ac20d6e633e90472b9e6e72b0177f7.png

https://www.answers.com › earth-science › What_is_the_reduction_of_c…
Reduction means a gain of electrons which is what non metals do in order to observe the octet rule For example elemental chlorine has an oxidation number of 0

https://socratic.org › questions › if-given-the-following-what-would-the-re…
Write out a number line The original E red was 0 8277 V when in reference to the Standard Hydrogen Electrode which is normally the one at 0 0000 V If we
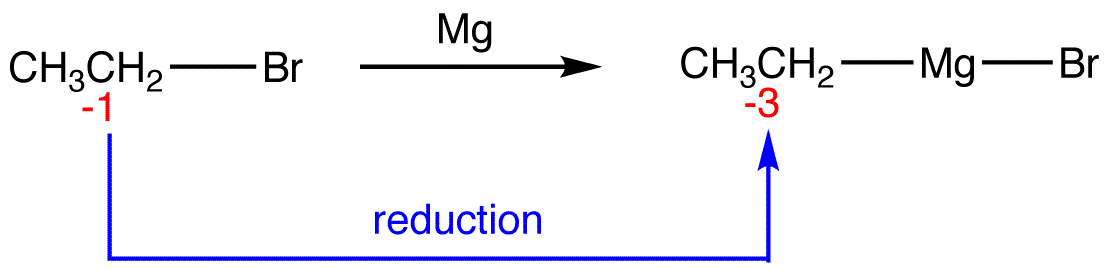
Reduction

State The Difference Between Oxidation And Reduction Brainly in
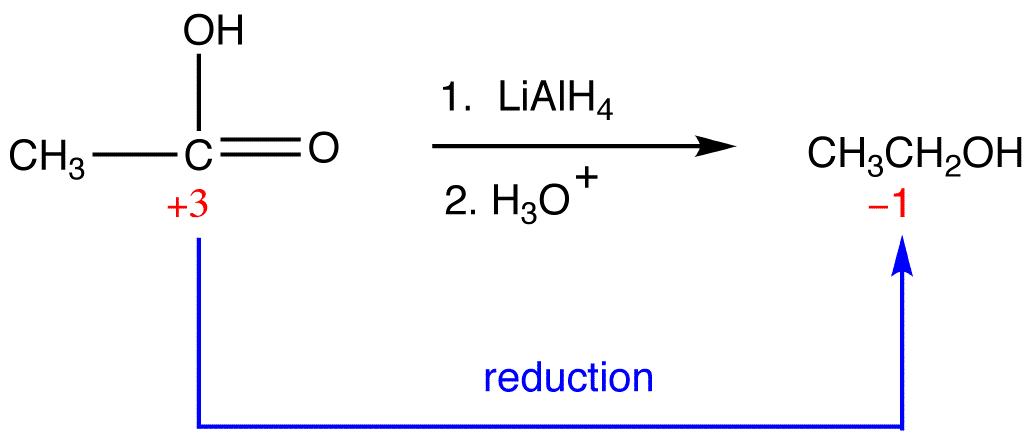
Reduction

Oxidation Reduction Redox Reactions Balancing Redox Reactions
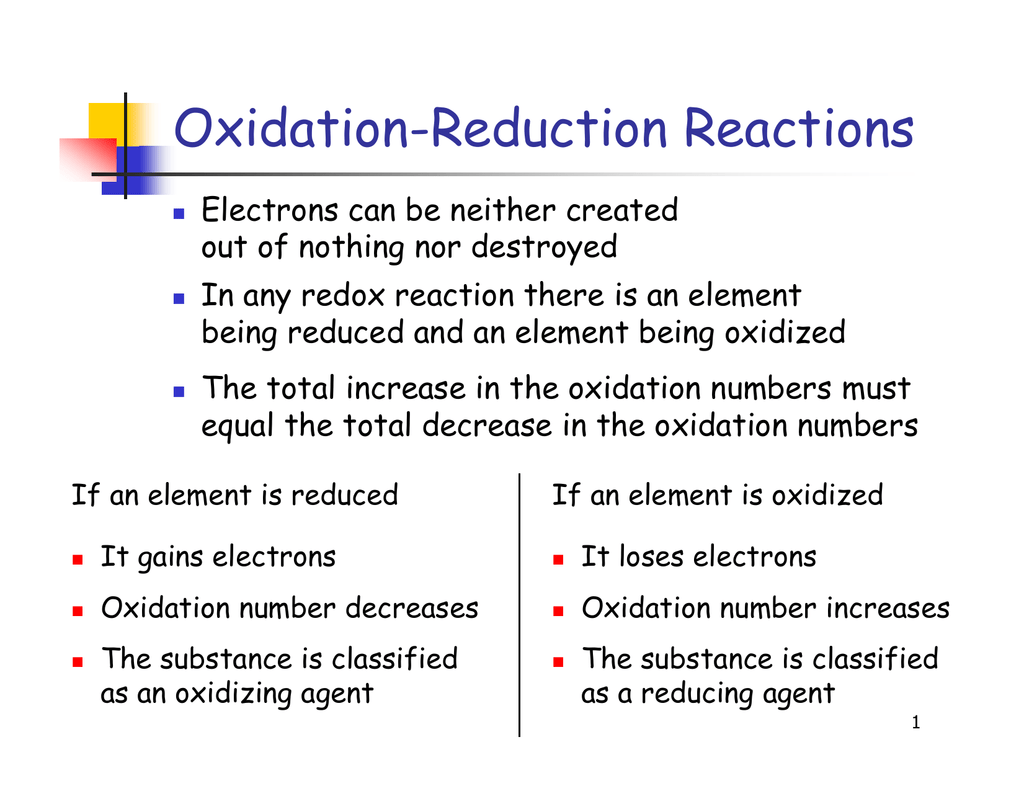
Types Of Oxidation
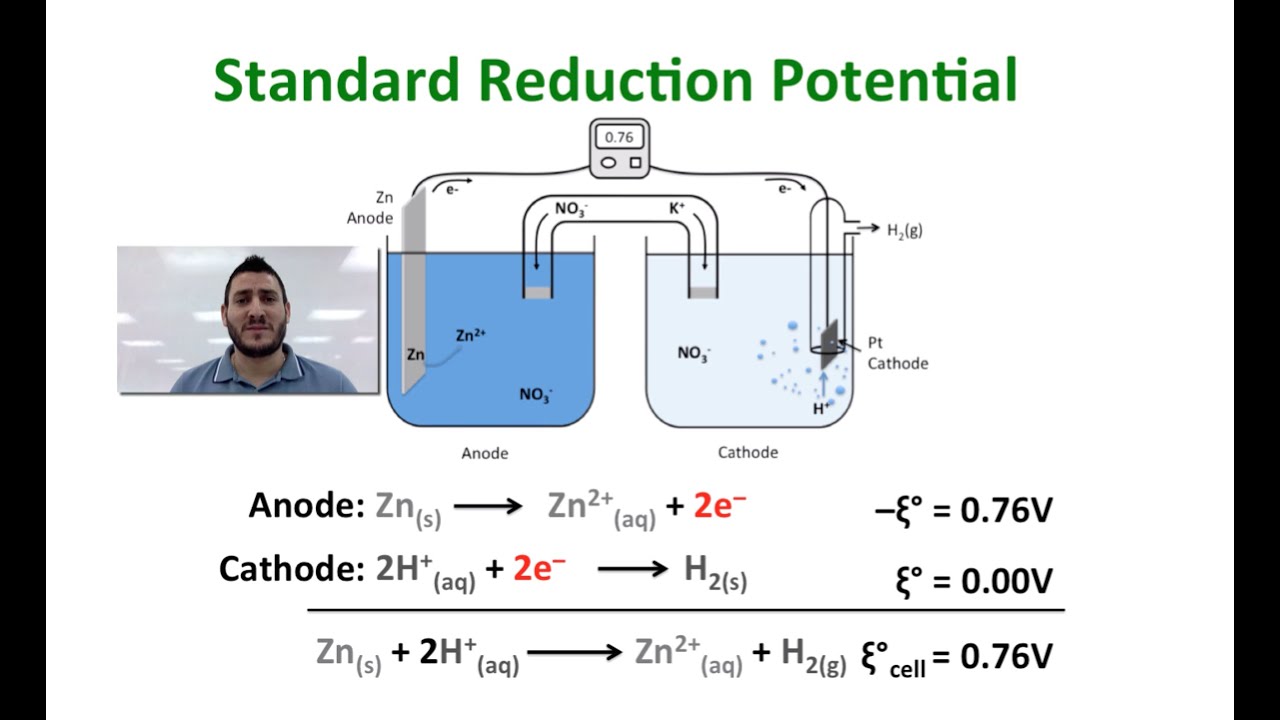
Electrochemistry The Standard Reduction Potential YouTube

Electrochemistry The Standard Reduction Potential YouTube
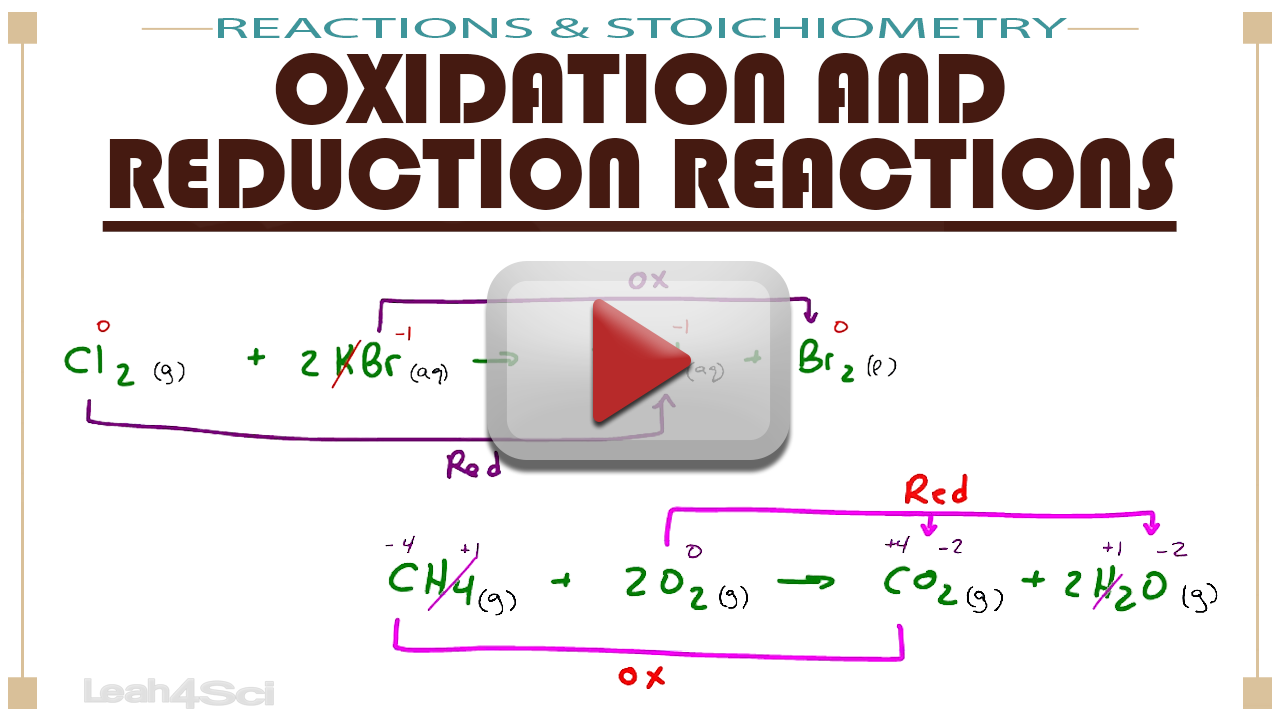
Reduction Chemistry
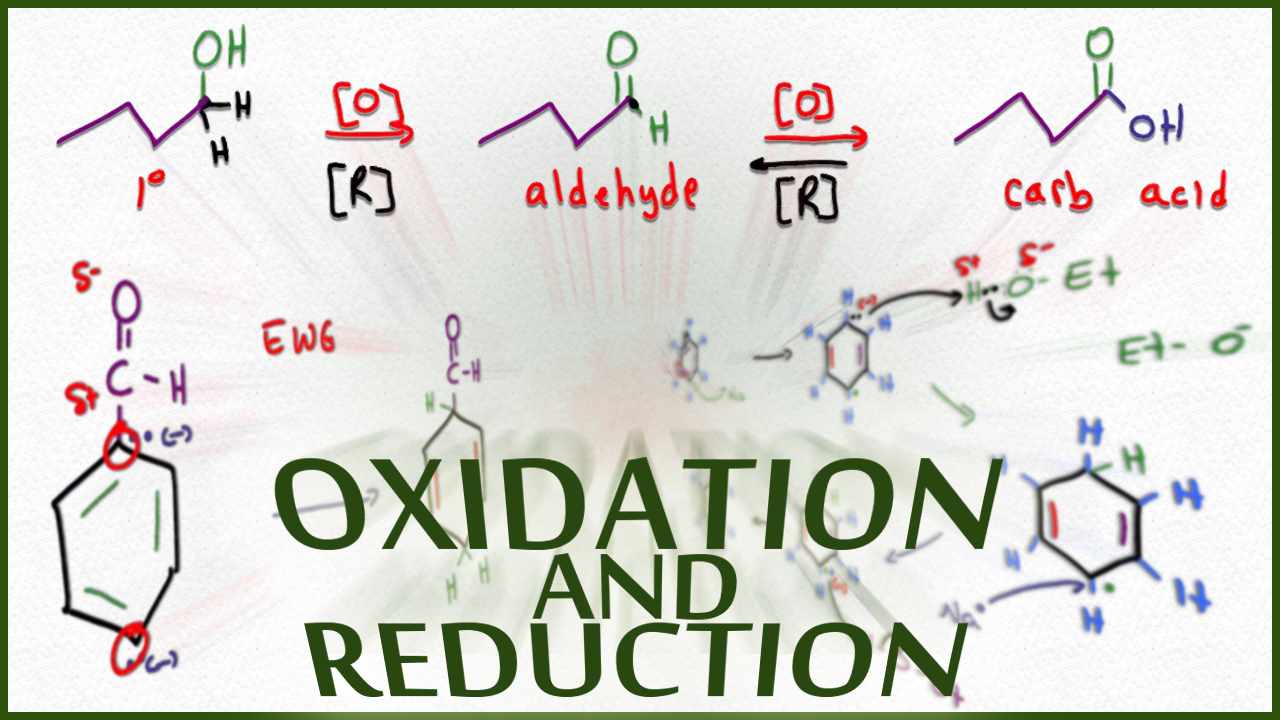
Oxidation Chemistry
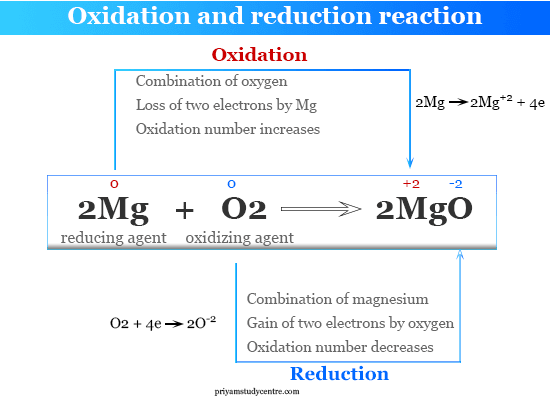
Oxidation Chemistry
What Is Reduction In Chemistry - [desc-12]